Products are selected by our editors, we may earn commission from links on this page.

In early August, Blusher covered the nationwide recall of hand soap products by the FDA due to sepsis risk, all from the same company. Before the month ended, on the 28th, the FDA expanded the list, following the company’s voluntary recall.
Manufactured by New Jersey–based DermaRite Industries, the affected items may be contaminated with Burkholderia cepacia, a bacterium that can cause severe infections and, in vulnerable populations, lead to life-threatening sepsis.
The recalled products were distributed nationwide in the United States and in Puerto Rico. Consumers are urged to stop using them immediately.
The Expanded Recall

What started as a limited recall has now grown into a much wider action by the FDA. Both the agency and the company have confirmed that more than 30 different DermaRite products, including soaps, sanitizers, deodorants, lotions, and wound care items, are affected. These products were not only shipped to healthcare facilities and long-term care centers but were also distributed through retail channels, meaning that ordinary consumers may have them at home.
Recalled Soaps, Cleansers, Washes, and Sanitizers

The FDA confirmed the following items are affected: 3-N-1 Body Wash, Shampoo & Perineal Cleanser, 4-N-1 No Rinse Wash Cream, Clean-N-Free Rinse-Free Body Wash, Shampoo & Perineal Cleanser, DermaKleen Antimicrobial Lotion Soap, DermaRain Body Wash & Shampoo, DermaVera Full Body Cleanser, Gel Rite Instant Gel Hand Sanitizer, Hand-E-Foam Foaming Hand Sanitizer, KleenFoam Antimicrobial Foam Soap, PeriFresh Incontinence Cleanser Spray, PeriGiene Antiseptic Cleanser, Renew Hair and Body Wash, Renew Full Body Wash & Shampoo, San-E-Foam Foaming Hand Sanitizer, TotalBath Body Wash & Shampoo, TotalFoam Body Wash & Shampoo, WhirlBath Body Wash & Shampoo,
These products are typically marketed for handwashing, bathing, and patient care settings.
Recalled Creams, Lotions, Protectants, and Other Products

In addition, the following DermaRite products are included in the recall: DermaCerin Moisturizing Cream, DemaDaily Moisturizing Lotion, DermaFungal Antifungal Cream, DemaKlenz Wound Cleanser, DermaMed Skin Protectant, DermaSarra External Analgesic, DermaSyn Hydrogel Wound Dressing, DermaVantage Moisturizing Lotion, Lantiseptic Skin Protectant, LubriSilk Lotion, PeriGuard Skin Protectant, Renew Dimethicone Skin Protectant (for diaper rash), Renew PeriProtect Skin Protectant, Renew Skin Repair Cream, UltraSure Antiperspirant & Deodorant.
These items include antifungal treatments, diaper rash creams, moisturizers, and deodorants, all of which fall under the recall.
The Contamination Risk
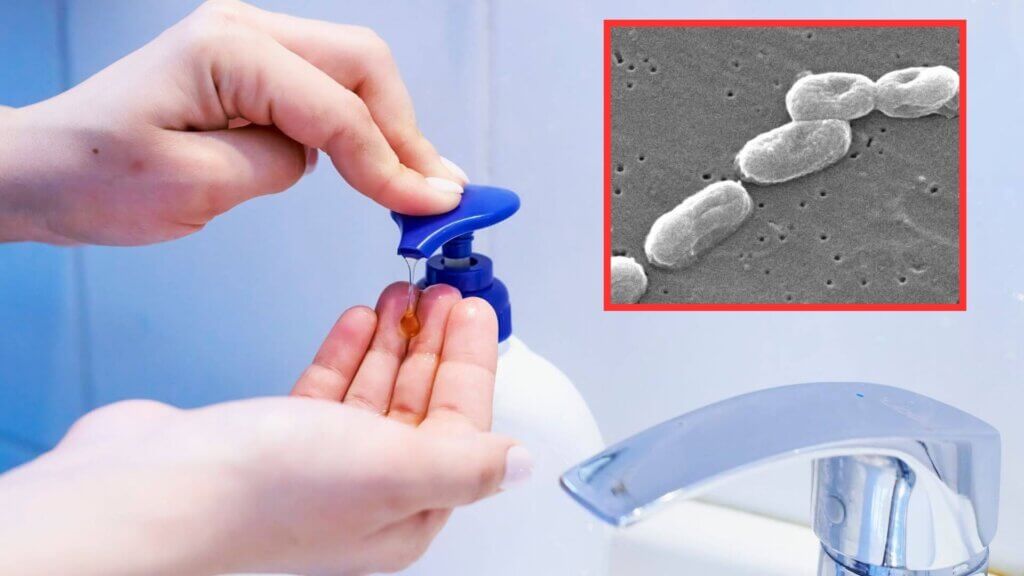
The concern is Burkholderia cepacia, a bacterium resilient in water-rich environments. While it may not harm most healthy individuals, it can cause pneumonia, bloodstream infections, and sepsis in immunocompromised people or those with chronic lung conditions.
Earlier Recall

This expansion builds on an earlier recall last month, when only four DermaRite products were pulled from the market: DermaKleen Antimicrobial Lotion Soap, DermaSarra External Analgesic, KleenFoam Antimicrobial Foam Soap, and PeriGiene. At that time, the FDA described the action as precautionary, but it warned that contaminated products could be dangerous for people with weakened immune systems.
DermaRite and FDA Actions

DermaRite says it has contacted its distributors, retailers, and healthcare partners to immediately stop selling or using the affected products. The company is instructing facilities to isolate and destroy recalled items in line with their disposal procedures. It has also set up a hotline and email address for customer questions.
For its part, the FDA is overseeing the recall and tracking reports of any health issues linked to the products. The agency has encouraged consumers not just to stop using recalled items, but also to file reports if they experience any infections or reactions.
What Consumers Should Do Now

Stop using recalled products immediately. Safely discard them according to local waste disposal guidelines. If you experience fever, respiratory symptoms, or other signs of infection, contact your doctor immediately.
Reporting Problems to the FDA

Consumers can report health issues linked to these products via the MedWatch Adverse Event Reporting Program: Online: fda.gov/medwatch/report.htm. Mail/Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
DermaRite has also provided a contact: Call Sedgwick at 888-943-5190, Monday through Friday, 8:00 am-5:00 pm EST, or email [email protected].
Why This Recall Matters

The sweeping recall underscores how even everyday hygiene products can present hidden health risks when contamination occurs. For consumers, it highlights the need to check recall notices regularly and to remain vigilant about the safety of products used daily.

